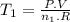Chemistry, 26.11.2019 06:31 kinziemadison12
Achemist prepares a sample of helium gas at a certain pressure, temperature and volume and then removes all but a fourth of the gas molecules (only a fourth remain). how must the temperature be changed (as a multiple of t1) to keep the pressure and the volume the same? a. t2=1/16t1b. t2=2t1c. t2=16t1d. t2= 1/2t1e. t2=4t1f. none of theseg. t2=1/4t1

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3


Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
Achemist prepares a sample of helium gas at a certain pressure, temperature and volume and then remo...
Questions in other subjects:



Mathematics, 01.04.2020 01:23




Mathematics, 01.04.2020 01:24