Given the standard enthalpy changes for the following two reactions:
(1) n2(g) + 2o2(g)2no2(...


Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:50, alainacorkell6472
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Questions in other subjects:

English, 17.02.2020 21:30



Mathematics, 17.02.2020 21:30






 ...... ΔH° = 66.4 kJ
...... ΔH° = 66.4 kJ
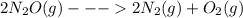 ......ΔH° = -164.2 kJ
......ΔH° = -164.2 kJ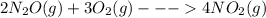 ......ΔH° = _________?
......ΔH° = _________?


