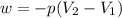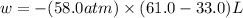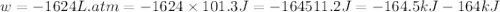Chemistry, 26.11.2019 04:31 tanakamkuzha
Question 22 a mixture of krypton and argon gas is expanded from a volume of 33.0l to a volume of 61.0l , while the pressure is held constant at 58.0atm . calculate the work done on the gas mixture. be sure your answer has the correct sign (positive or negative) and the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, btcastongia
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3



Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1
You know the right answer?
Question 22 a mixture of krypton and argon gas is expanded from a volume of 33.0l to a volume of 61....
Questions in other subjects:


Physics, 09.11.2019 17:31



Mathematics, 09.11.2019 17:31





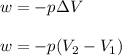
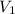 = initial volume = 33.0 L
= initial volume = 33.0 L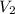 = final volume = 61.0 L
= final volume = 61.0 L