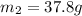Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
A32.4 g iron rod, initially at 21.6 ∘c, is submerged into an unknown mass of water at 63.1 ∘c, in an...
Questions in other subjects:









Physics, 13.04.2021 16:10

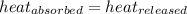
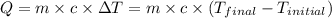
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0390/9808/09236.png) .................(1)
.................(1)
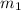 = mass of iron rod = 32.4 g
= mass of iron rod = 32.4 g
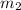 = mass of water = ?
= mass of water = ?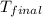 = final temperature =
= final temperature = 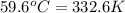
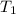 = temperature of iron rod =
= temperature of iron rod = 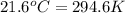
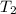 = temperature of water =
= temperature of water = 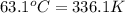
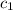 = specific heat of iron rod =
= specific heat of iron rod = 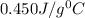
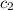 = specific heat of water=
= specific heat of water= 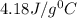
![32.4\times 0.450\times (332.6-294.6)=-[m_2\times 4.18\times (332.6-336.1)]](/tpl/images/0390/9808/cb847.png)