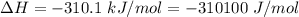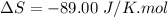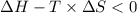Chemistry, 26.11.2019 03:31 Rileyb101207
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reaction in which δh = −310.1 kj/mol and δs = −89.00 j/k · mol, determine the temperature (in °c) below which the reaction is spontaneous.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reactio...
Questions in other subjects:

Mathematics, 24.01.2020 22:31

Mathematics, 24.01.2020 22:31

History, 24.01.2020 22:31

Mathematics, 24.01.2020 22:31




Mathematics, 24.01.2020 22:31


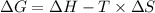
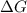 is the change in the Gibbs free energy.
is the change in the Gibbs free energy.
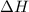 is the enthalpy change of the reaction.
is the enthalpy change of the reaction.
 is the change in entropy.
is the change in entropy.