Chemistry, 26.11.2019 03:31 ashtonrieper1132
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 17. g of octane is mixed with 93.0 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1
You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . s...
Questions in other subjects:



History, 02.04.2021 03:30





Mathematics, 02.04.2021 03:30

Physics, 02.04.2021 03:30

English, 02.04.2021 03:30

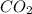
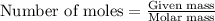
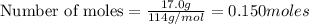
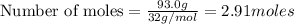
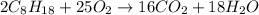
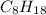 require 25 moles of
require 25 moles of 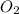
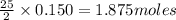 of
of 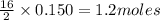 of
of