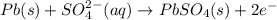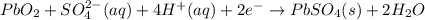Chemistry, 26.11.2019 03:31 giannimaraj711
In a lead-acid battery, the electrodes are consumed. in this battery, select one: a. the anode is pbo2. b. the cathode is pb. c. the cathode is pbso4. d. the anode is pbso4. e. the anode is pb.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
In a lead-acid battery, the electrodes are consumed. in this battery, select one: a. the anode is p...
Questions in other subjects:

Mathematics, 26.09.2019 03:30

History, 26.09.2019 03:30


Mathematics, 26.09.2019 03:30


History, 26.09.2019 03:30

Mathematics, 26.09.2019 03:30


English, 26.09.2019 03:30