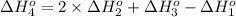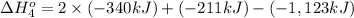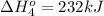Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 23.06.2019 01:30, kcarstensen59070
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 09:30, crawford184232323234
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2
You know the right answer?
Calculate the enthalpy for this reaction: 2c(s) + h2(g) > c2h2(g) δh° = kj given the following...
Questions in other subjects:










 ...[1]
...[1]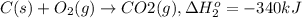 ..[2]
..[2]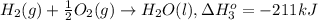 ..[3]
..[3]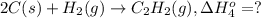 ..[4]
..[4]