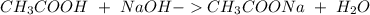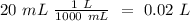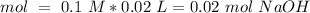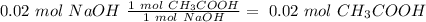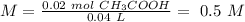Chemistry, 26.11.2019 01:31 jsjsjsskakwkowwj
40.0 ml of an acetic acid of unknown concentration is titrated with 0.100 m naoh. after 20.0 ml of the base solution has been added, the ph in the titration flask is 5.10. what was the concentration of the original acetic acid solution? (ka(ch3cooh) = 1.8 × 10–5)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:50, kyleighmarie05
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3



Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
40.0 ml of an acetic acid of unknown concentration is titrated with 0.100 m naoh. after 20.0 ml of t...
Questions in other subjects:


Mathematics, 01.04.2021 18:50



Mathematics, 01.04.2021 18:50



Mathematics, 01.04.2021 18:50


Mathematics, 01.04.2021 18:50