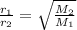Chemistry, 26.11.2019 01:31 jeovontamarley
The rate of effusion of a gas, r, is inversely proportional to the square root of its molar mass, m. the relative rate of two different gases is expressed as r1r2=m2m1−−−√ where r1 and r2 are the effusion rates of two gases and m1 and m2 are their respective molar masses. part a in an effusion experiment, it was determined that nitrogen gas, n2, effused at a rate 1.812 times faster than an unknown gas. what is the molar mass of the unknown gas? express your answer to four significant figures and include the appropriate units.

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
The rate of effusion of a gas, r, is inversely proportional to the square root of its molar mass, m....
Questions in other subjects:



Computers and Technology, 26.01.2021 17:20





History, 26.01.2021 17:20

Chemistry, 26.01.2021 17:20

Mathematics, 26.01.2021 17:20