
Chemistry, 26.11.2019 01:31 billlyyyyyyyyyy
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. 9.00 x 102 kj 64.1 kj -9.00 x 102 kj -64.1 kj -65.9 kj

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 07:00, ceeejay0621
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is per...
Questions in other subjects:

Mathematics, 28.06.2020 15:01

Mathematics, 28.06.2020 15:01

Mathematics, 28.06.2020 15:01


Mathematics, 28.06.2020 15:01


Mathematics, 28.06.2020 15:01



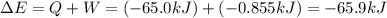

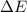 =Change in internal energy
=Change in internal energy
 {Work is done by the system is negative as the final volume is greater than initial volume}
{Work is done by the system is negative as the final volume is greater than initial volume}




