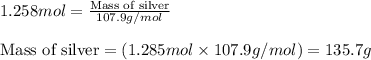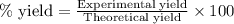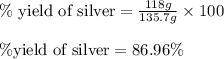Chemistry, 26.11.2019 00:31 agarcia24101993
When 40.0 g of copper are reacted with silver nitrate solution cu + 2 agno3 --> cu(no3)2 + 2 ag 118 g of silver are obtained. what is the percent yield of silver? molar mass of silver = 107.9 g, molar mass of copper = 63.55 g

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
When 40.0 g of copper are reacted with silver nitrate solution cu + 2 agno3 --> cu(no3)2 + 2 ag...
Questions in other subjects:

Mathematics, 29.10.2020 01:00

Mathematics, 29.10.2020 01:00


Chemistry, 29.10.2020 01:00

Mathematics, 29.10.2020 01:00



Biology, 29.10.2020 01:00


History, 29.10.2020 01:00

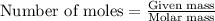 .....(1)
.....(1)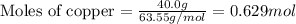
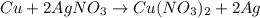
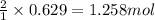 of silver
of silver