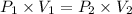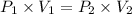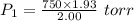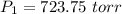Chemistry, 25.11.2019 22:31 adantrujillo1234
A2.00-l sample of was collected over water at a total pressure of 750. torr and 26 °c. when the was dried (water vapor removed), the gas had a volume of 1.93 l at 26 °c and 750. torr. calculate the vapor pressure of water at 26 °c.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, yselahernandez02
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 21.06.2019 19:00, lazavionadams81
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1
You know the right answer?
A2.00-l sample of was collected over water at a total pressure of 750. torr and 26 °c. when the was...
Questions in other subjects:

History, 20.03.2021 06:10


History, 20.03.2021 06:10

Mathematics, 20.03.2021 06:10



Health, 20.03.2021 06:10

Mathematics, 20.03.2021 06:10


World Languages, 20.03.2021 06:10