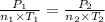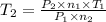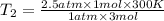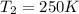Chemistry, 25.11.2019 21:31 ErrorNameTaken505
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide. the original pressure and temperature in the flask is 1.0 atm and 300. k. all of the solid carbon dioxide sublimes. the final pressure in the flask is 2.5 atm. what is the final temperature? assume the solid carbon dioxide takes up negligible volume. a. 150 kb. 200 kc. 250 kd. 300 ke. 400 k

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, lazavionadams81
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1


Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide....
Questions in other subjects:


Chemistry, 29.03.2021 20:10

Mathematics, 29.03.2021 20:10


Mathematics, 29.03.2021 20:10

Mathematics, 29.03.2021 20:10



Mathematics, 29.03.2021 20:10

Mathematics, 29.03.2021 20:10