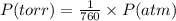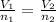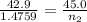Chemistry, 25.11.2019 21:31 ayoismeisalex
A42.9 l gas sample containing 1.50 moles of oxygen at 25.0 celcius exerts a pressure of 64.0.0 torr. the volume increases to 45.0 l when additional oxygen gas is added to the sample at constant temperature and pressure. how many moles have been added? what mass of gas was added?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 23.06.2019 00:50, alainacorkell6472
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 08:00, george27212
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
A42.9 l gas sample containing 1.50 moles of oxygen at 25.0 celcius exerts a pressure of 64.0.0 torr....
Questions in other subjects:

History, 27.02.2021 03:10



Mathematics, 27.02.2021 03:10




Mathematics, 27.02.2021 03:10


Mathematics, 27.02.2021 03:10