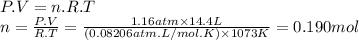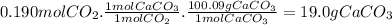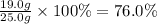Chemistry, 25.11.2019 21:31 zanaplen27
For the reaction below, kp = 1.16 at 800.°c. caco3(s) equilibrium reaction arrow cao(s) + co2(g) if a 25.0-g sample of caco3 is put into a 14.4 l container and heated to 800°c, what percentage by mass of the caco3 will react to reach equilibrium?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2

Chemistry, 22.06.2019 05:30, tifftiff22
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
For the reaction below, kp = 1.16 at 800.°c. caco3(s) equilibrium reaction arrow cao(s) + co2(g) if...
Questions in other subjects:

Mathematics, 08.12.2020 01:40

Mathematics, 08.12.2020 01:40



Mathematics, 08.12.2020 01:40




History, 08.12.2020 01:40