
Chemistry, 25.11.2019 20:31 jakobrobinette
Kemmi major does some experimental work on the combustion of sucrose: c12h22o11(s) 12 o2(g) → 12 co2(g) 11 h2o(g) she burns a 0.05392 g pellet of sucrose in a bomb calorimeter with excess oxygen. she determines the qrxn to be –916.6 j for the reaction. calculate the ∆h value for the combustion reaction. (round the answer to 3 significant digits, units of kj, pay attention to positive or negative.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 21.06.2019 22:40, wiltseliz4800
What does the process of natural selection involve
Answers: 1

Chemistry, 22.06.2019 07:20, rex1578
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1
You know the right answer?
Kemmi major does some experimental work on the combustion of sucrose: c12h22o11(s) 12 o2(g) → 12 co...
Questions in other subjects:





Mathematics, 03.07.2019 18:20





Physics, 03.07.2019 18:20

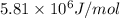
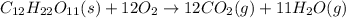
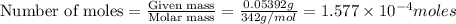
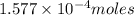 of sucrose releases = 916.6 J of heat
of sucrose releases = 916.6 J of heat
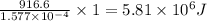 of heat
of heat


