Consider the following reversible reaction.
what is the equilibrium constant expression...

Chemistry, 24.11.2019 08:31 vapelordcarl69
Consider the following reversible reaction.
what is the equilibrium constant expression for the given system?
1st pic is equation, 2nd is a, 3rd is b, 4th is c, and 5th is d.
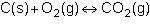
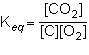
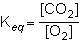
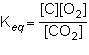
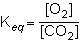

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
Questions in other subjects:

Social Studies, 12.01.2021 17:40



Chemistry, 12.01.2021 17:40






Computers and Technology, 12.01.2021 17:40



