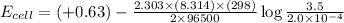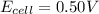Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
The standard cell potential (e°cell) for the reaction below is +0.63 v. the cell potential for this...
Questions in other subjects:

SAT, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00

Business, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00

Biology, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00

World Languages, 15.02.2022 14:00


Mathematics, 15.02.2022 14:00

Mathematics, 15.02.2022 14:00




![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]}{[Pb^{2+}]}](/tpl/images/0387/6329/1472e.png)
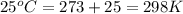
 = standard electrode potential of the cell = +0.63 V
= standard electrode potential of the cell = +0.63 V = cell potential for the reaction = ?
= cell potential for the reaction = ?![[Zn^{2+}]](/tpl/images/0387/6329/9c01a.png) = 3.5 M
= 3.5 M![[Pb^{2+}]](/tpl/images/0387/6329/0acfd.png) =
=