
Naoh(s) dissolves readily in water to form na+(aq) and oh-(aq). if 35.1 g of naoh is dissolved in 199 g of water in a coffee cup calorimeter, the temperature of the resulting solution increases from 23.0 oc to 62.6 oc. calculate the enthalpy change per mole of naoh dissolved. assume the specific heat capacity of the solution is 4.18 j/oc*g.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 09:30, sharmadaman641
What is the best describtion of the side of the moon that faces earth?
Answers: 2
You know the right answer?
Naoh(s) dissolves readily in water to form na+(aq) and oh-(aq). if 35.1 g of naoh is dissolved in 19...
Questions in other subjects:

Chemistry, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20

Chemistry, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20



Mathematics, 17.02.2021 22:20


Mathematics, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20

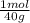 = 0,878 moles
= 0,878 moles

