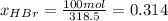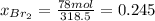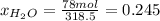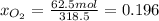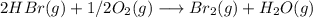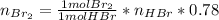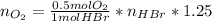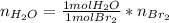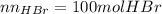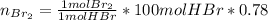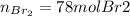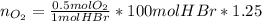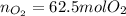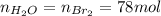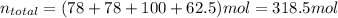Chemistry, 23.11.2019 04:31 rodrickahammonds
Bromine (br2) is produced by reacting hbr with o2, with water as a byproduct. the o2 is part of an air (21 mol % o2, 79 mol % n2) feed stream that is flowing sufficiently fast to provide 25% excess oxygen ("excess" has a precise meaning in process analysis: in this case there is 25% more oxygen than the amount needed to completely react with the limiting reactant). the fractional conversion of hbr is 78%.
a) show the degree of freedom analysis. be as specific as possible about labeling the unknowns and completely write out all of the independent equations.
b) calculate the composition (mole fractions) of the product stream.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
Bromine (br2) is produced by reacting hbr with o2, with water as a byproduct. the o2 is part of an a...
Questions in other subjects:



Mathematics, 18.03.2020 21:36


Biology, 18.03.2020 21:36

Mathematics, 18.03.2020 21:36

Mathematics, 18.03.2020 21:37



English, 18.03.2020 21:37