
For a beaker containing the reaction below that is at equilibrium, using lechatlier's principle, predict which way the equilibrium conditions will shift (type in left, right, or no change) if silver chloride was added to a beaker.
agno3 (aq) + nacl (aq) → agcl (s) + nano3 (aq)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, whrjegt4jrnfdvj
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 21:30, sarah192002
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 06:00, BigGirlsTheBest
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2

Chemistry, 23.06.2019 08:00, johngayden46
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2
You know the right answer?
For a beaker containing the reaction below that is at equilibrium, using lechatlier's principle, pre...
Questions in other subjects:

Physics, 12.08.2021 18:50





Mathematics, 12.08.2021 18:50


Mathematics, 12.08.2021 18:50


Mathematics, 12.08.2021 18:50

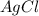 is added.
is added.

