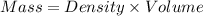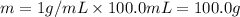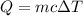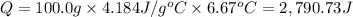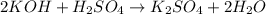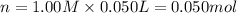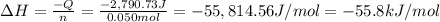When 50.0 ml of 0.500 m h2so4 is added to 50.0 ml of 1.00 m koh in a coffee cup calorimeter, the temperature of the solution rises from 25.10°c to 31.77°c. calculate δh of this reaction (in kj/mol koh). assume that the total volume is the sum of the individual volumes and that the density and specific heat capacity of the solution are the same as that for pure water (c = 4.184j/g°c).a. -112 kj/mol kohb. -2.79 kj/mol kohc. -74.9 kj/mol kohd. -1.86 kj/mol kohe. -55.8 kj/mol koh

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, Gghbhgy4809
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 23.06.2019 03:00, BeeShyanne
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1

Chemistry, 23.06.2019 07:30, lucas2020197
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
When 50.0 ml of 0.500 m h2so4 is added to 50.0 ml of 1.00 m koh in a coffee cup calorimeter, the tem...
Questions in other subjects:


Computers and Technology, 10.08.2021 22:00




Mathematics, 10.08.2021 22:00


English, 10.08.2021 22:00


Mathematics, 10.08.2021 22:00