
Chemistry, 30.12.2019 16:31 issaaamiaaa15
When the compound, na2s, is mixed with water, the na2s is:
soluble because all na+1 compounds are soluble in water
insoluble because most na+1 compounds are insoluble in water
soluble because all s−2 compounds are soluble in water
insoluble because there are no water soluble s−2 compounds

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
When the compound, na2s, is mixed with water, the na2s is:
soluble because all na+1 com...
soluble because all na+1 com...
Questions in other subjects:


Mathematics, 06.05.2020 06:42

Mathematics, 06.05.2020 06:42

Mathematics, 06.05.2020 06:42

Mathematics, 06.05.2020 06:42


English, 06.05.2020 06:42



Mathematics, 06.05.2020 06:42

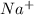 by losing an electron and sulfur changes into
by losing an electron and sulfur changes into 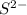 by gain of two electrons from two sodium atoms.
by gain of two electrons from two sodium atoms.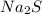 , is mixed with water, the
, is mixed with water, the 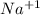 compounds are soluble in water.
compounds are soluble in water.

