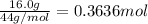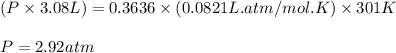Chemistry, 22.11.2019 23:31 oliviajewelwilliams
Cyclists sometimes use pressurized carbon dioxide inflators to inflate a bicycle tire in the event of a flat. these inflators use metal cartridges that contain 16.0 g of carbon dioxide.
at 301 k , to what pressure (in psi) can the carbon dioxide in the cartridge inflate a 3.08 l mountain bike tire?
(note: the gauge pressure is the difference between the total pressure and atmospheric pressure. in this case, assume that atmospheric pressure is 14.7 psi.)
express your answer to three significant figures with the appropriate units.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
Cyclists sometimes use pressurized carbon dioxide inflators to inflate a bicycle tire in the event o...
Questions in other subjects:


Mathematics, 22.04.2021 18:30

Mathematics, 22.04.2021 18:30




Mathematics, 22.04.2021 18:30



Mathematics, 22.04.2021 18:30