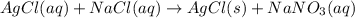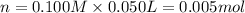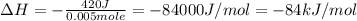Chemistry, 22.11.2019 19:31 jadabecute3739
 + nacl(aq) \rightarrow agcl(s)\ +\ nano _3(aq)) in an experiment a student mixes a 50.0 ml sample of 0.100 m agno₃ (aq) with a 50.0 ml sample of 0.100 m nacl(aq) at 20.0°c in a coffee-cup calorimeter. which of the following is the enthalpy change of the precipitation reaction represented above if the final temperature of the mixture is 21.0°c? (assume that the total mass of the mixture is 100. g and that the specific heat capacity of the mixture is 4.2 j/(g.° (a) -84 kj/mol, (b) -0.42 kj/mol (c) 0.42 kj/molu (d) 84 kj/molpx
in an experiment a student mixes a 50.0 ml sample of 0.100 m agno₃ (aq) with a 50.0 ml sample of 0.100 m nacl(aq) at 20.0°c in a coffee-cup calorimeter. which of the following is the enthalpy change of the precipitation reaction represented above if the final temperature of the mixture is 21.0°c? (assume that the total mass of the mixture is 100. g and that the specific heat capacity of the mixture is 4.2 j/(g.° (a) -84 kj/mol, (b) -0.42 kj/mol (c) 0.42 kj/molu (d) 84 kj/molpx

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 23.06.2019 09:20, taylorannsalazar
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
[tex]agno _3(aq) + nacl(aq) \rightarrow agcl(s)\ +\ nano _3(aq)[/tex]in an experiment a student mixe...
Questions in other subjects:


Mathematics, 26.02.2021 22:20

Physics, 26.02.2021 22:20




Mathematics, 26.02.2021 22:20



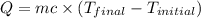
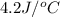
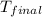 = final temperature =
= final temperature = 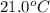
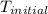 = initial temperature =
= initial temperature = 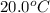
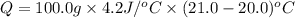
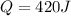
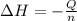
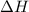 = enthalpy change = ?
= enthalpy change = ?