Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

You know the right answer?
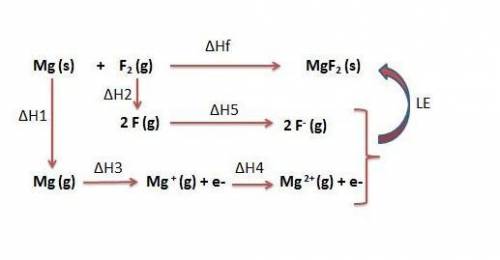
Use the following to calculate h°lattice of mgf2. mg(s) mg(g) h° = 148 kj f2(g) 2 f(g) h° = 159 kj m...
Questions in other subjects:

Mathematics, 05.12.2020 14:00



English, 05.12.2020 14:00


Social Studies, 05.12.2020 14:00




Mathematics, 05.12.2020 14:00