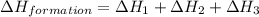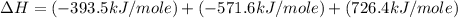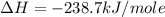Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 --> co2(g) latex: \deltaδh° = –393.5 kj/mol h2(g) + (1/2)o2 --> h2o(l) latex: \deltaδh° = –285.8 kj/mol ch3oh(l) + (3/2)o2(g) --> co2(g) + 2h2o(l) latex: \deltaδh° = –726.4 kj/mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

You know the right answer?
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following infor...
Questions in other subjects:

Mathematics, 12.12.2020 16:10


English, 12.12.2020 16:10


Mathematics, 12.12.2020 16:10

Chemistry, 12.12.2020 16:10



Mathematics, 12.12.2020 16:10

 will be,
will be,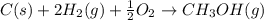
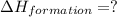
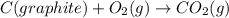
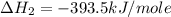
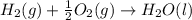
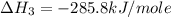
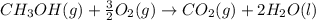
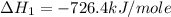
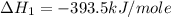
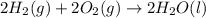
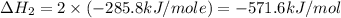
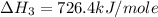
 will be,
will be,