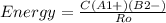Chemistry, 22.11.2019 01:31 makaylahunt
An ionic bond is formed between a cation a1 and an anion b2. how would the energy of the ionic bond [see equation (9.2)] be affected by the following changes? (a) doubling the radius of a1, (b) tripling the charge on a1, (c) doubling the charges on a1 and b2, (d) decreasing the radii of a1 and b2 to half their original values.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
An ionic bond is formed between a cation a1 and an anion b2. how would the energy of the ionic bond...
Questions in other subjects:



Mathematics, 16.04.2021 17:40

Chemistry, 16.04.2021 17:40



Physics, 16.04.2021 17:40

Arts, 16.04.2021 17:40

History, 16.04.2021 17:40

Social Studies, 16.04.2021 17:40