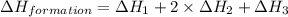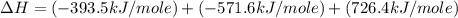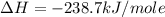Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2
You know the right answer?
Given the following heats of combustion. ch3oh(l) + 3/2 o2(g) co2(g) + 2 h2o(l) δh°rxn = -726.4 kj c...
Questions in other subjects:


Mathematics, 26.06.2019 19:30








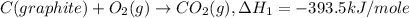 ..[1]
..[1]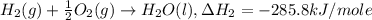 ..[2]
..[2]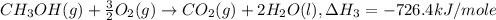 ..[3]
..[3]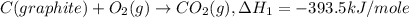 ..[1]
..[1]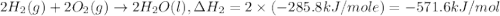 ..[2]
..[2]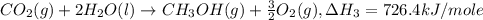 [3]
[3]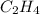 will be,
will be,