
Chemistry, 21.11.2019 23:31 katielloyd
A1.800 g sample of octane, c8h18, is burned a calorimeter whose total heat capacity is 12.66 kj/°c. if the temperature of the calorimeter increased from 22.36 °c to 28.78 °c, then what is the δh for the combustion of one mole of octane? do not add the unit in the answer.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 02:30, ggpro4life3000
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
A1.800 g sample of octane, c8h18, is burned a calorimeter whose total heat capacity is 12.66 kj/°c....
Questions in other subjects:




Business, 15.12.2021 19:30

Social Studies, 15.12.2021 19:30

Geography, 15.12.2021 19:30

Mathematics, 15.12.2021 19:30




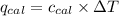
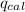 = Heat gained by bomb calorimeter
= Heat gained by bomb calorimeter  =Heat capacity of bomb calorimeter=12.66 kJ/°C
=Heat capacity of bomb calorimeter=12.66 kJ/°C

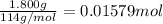
 of heat
of heat for the combustion of one mole of octane is
for the combustion of one mole of octane is 

