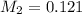Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
If 0.290g of fas is dissolved in 10ml of di water and is titrated to equivalence with 12.23ml kmno4,...
Questions in other subjects:

Arts, 06.11.2020 03:50


History, 06.11.2020 03:50

Mathematics, 06.11.2020 03:50


Social Studies, 06.11.2020 03:50

English, 06.11.2020 03:50



Mathematics, 06.11.2020 03:50

 is 0.121 M
is 0.121 M
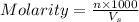

 = volume of solution = 10 ml
= volume of solution = 10 ml
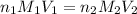
 = molarity of
= molarity of 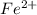 solution = 0.074 M
solution = 0.074 M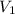 = volume of
= volume of 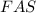 solution = 10 ml
solution = 10 ml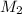 = molarity of
= molarity of  = volume of
= volume of  = valency of
= valency of  = valency of
= valency of