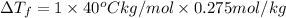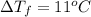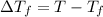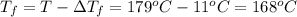Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
Assume the molality of isoborneol in your product is 0.275 mol/kg. what is the melting point of your...
Questions in other subjects:


Business, 27.11.2020 14:00

Physics, 27.11.2020 14:00

Mathematics, 27.11.2020 14:00

English, 27.11.2020 14:00


Mathematics, 27.11.2020 14:00

Engineering, 27.11.2020 14:00

Mathematics, 27.11.2020 14:00

Chemistry, 27.11.2020 14:00

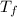 = ?
= ?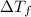
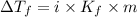
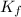 = The freezing point depression constant
= The freezing point depression constant