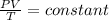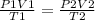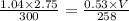Chemistry, 21.11.2019 22:31 darnellgee298
Ahelium balloon with a volume of 2.75 l and pressure of 1.04 atm and 27 °c flies in the sky and reaches an altitude where the temperature is -15 °c and the pressure is 0.530 atm. calculate the final volume of the balloon

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
You know the right answer?
Ahelium balloon with a volume of 2.75 l and pressure of 1.04 atm and 27 °c flies in the sky and reac...
Questions in other subjects:

Spanish, 24.04.2021 01:00






Mathematics, 24.04.2021 01:00

History, 24.04.2021 01:00