
Two nonpolar organic liquids, hexane (c_6h_14) and heptane (c_7h_16) are mixed. hexane and heptane are miscible with each other in all proportions. in making a solution of them, is the entropy of the system increased, decreased, or close to zero, compared to the separate pure liquids?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Homepage10
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1

Chemistry, 21.06.2019 23:30, lylessd423
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 09:00, yogibear5806
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2
You know the right answer?
Two nonpolar organic liquids, hexane (c_6h_14) and heptane (c_7h_16) are mixed. hexane and heptane a...
Questions in other subjects:



English, 23.01.2020 06:31

Mathematics, 23.01.2020 06:31

Mathematics, 23.01.2020 06:31

Business, 23.01.2020 06:31

History, 23.01.2020 06:31


Mathematics, 23.01.2020 06:31

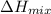 is nearly zero)
is nearly zero)

