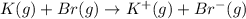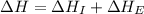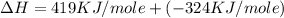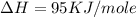Chemistry, 21.11.2019 21:31 shongmadi77
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following ionization energy (ie) and electron affinity (ea) values (hint: should one be negative for the reaction? ) ie ea k: 419 kj/mol 48 kj/mol br: 1140 kj/mol 324 kj/mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 06:00, girlwonder326
+= + + balance the equation on coefficients, ex. 1,1,1
Answers: 2

Chemistry, 23.06.2019 10:30, taniyahbenyamin2
What’s the physical properties in calcium chloride
Answers: 1
You know the right answer?
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following io...
Questions in other subjects:




Business, 28.07.2019 03:30






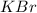 :
: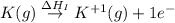
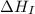 = ionization energy of potassium = 419 kJ/mol
= ionization energy of potassium = 419 kJ/mol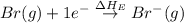
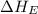 = electron affinity energy of bromine = -324 kJ/mol
= electron affinity energy of bromine = -324 kJ/mol