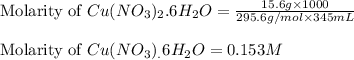Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 04:40, laurabwhiddon
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

Chemistry, 23.06.2019 10:50, uh8hardiek
Mach the labels with the symbols on the weather map
Answers: 2
You know the right answer?
An aqueous solution is prepared by dissolving 15.6 g of cu(no3)2 ⋅ 6 h2o in water and diluting to 34...
Questions in other subjects:

Biology, 24.05.2021 01:00

Mathematics, 24.05.2021 01:00

Mathematics, 24.05.2021 01:00


History, 24.05.2021 01:00



English, 24.05.2021 01:00


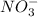 ions in the solution is 0.306 M
ions in the solution is 0.306 M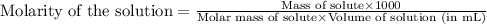
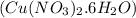 = 15.6 g
= 15.6 g