Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
Aflask contains 0.25 mole of so2(g), 0.50 mole of ch4(g), and 0.50 mole of o2(g). the total pressure...
Questions in other subjects:




Health, 25.11.2021 18:50

Physics, 25.11.2021 18:50

Physics, 25.11.2021 18:50


Mathematics, 25.11.2021 18:50

Computers and Technology, 25.11.2021 18:50

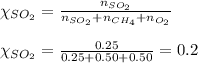

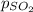 = partial pressure of sulfur dioxide gas
= partial pressure of sulfur dioxide gas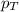 = total pressure = 800 mmHg
= total pressure = 800 mmHg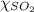 = mole fraction of sulfur dioxide = 0.2
= mole fraction of sulfur dioxide = 0.2