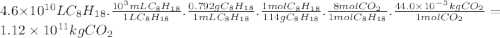Chemistry, 21.11.2019 20:31 jacksonyodell4184
Assuming that gasoline is 100% isooctane, that isooctane burns to produce only co2 and h2o, and that the density of isooctane is 0.792 g/ml, what mass of co2 (in kilograms) is produced each year by the annual u. s. gasoline consumption of 4.6×1010l?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Assuming that gasoline is 100% isooctane, that isooctane burns to produce only co2 and h2o, and that...
Questions in other subjects:

Mathematics, 25.11.2021 14:00


Mathematics, 25.11.2021 14:00


Social Studies, 25.11.2021 14:00