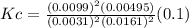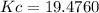Chemistry, 21.11.2019 09:31 thatonestudent2271
A100 ml reaction vessel initially contains 2.60×10^-2 moles of no and 1.30×10^-2 moles of h2. at equilibrium the concentration of no in the vessel is 0.161m. at equilibrium the vessel also contains n2, h2o, and h2. what is the value of the equilibrium constant for kc for the following reaction?
2h2 (g) + 2no(g) < —> 2h2o (g) + n2 (g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:40, monkey2865
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3


Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
A100 ml reaction vessel initially contains 2.60×10^-2 moles of no and 1.30×10^-2 moles of h2. at equ...
Questions in other subjects:


Computers and Technology, 29.12.2020 14:00

History, 29.12.2020 14:00

Mathematics, 29.12.2020 14:00


Computers and Technology, 29.12.2020 14:00




Mathematics, 29.12.2020 14:00

 ml
ml moles
moles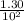 moles
moles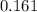 M
M
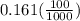
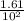 moles
moles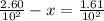
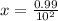
![Kc=\frac{[H2O]^2[N2]}{[H2]^2[NO]^2} (volume of vesselin litre)](/tpl/images/0384/4648/de870.png)