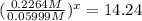At 593k a particular decomposition’s rate constant had a value of 5.21×10−4 and at 673k the same reaction’s rate constant was 7.42×10−3. it was noticed that when the reactant’s initial concentration was 0.2264 m (with a 593k reaction temperature), the initial reaction rate was identical to the initial rate when the decomposition was run at 673k with an initial reactant concentration of 0.05999 m. recall that rate laws have the form rate = k [a]x and, showing work, determine the order of the decomposition reaction.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 01:00, Johnson926
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
At 593k a particular decomposition’s rate constant had a value of 5.21×10−4 and at 673k the same rea...
Questions in other subjects:



Social Studies, 30.11.2021 07:50



Mathematics, 30.11.2021 07:50




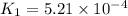
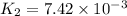
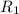
![R_1=K_1\times [A]^x](/tpl/images/0384/0720/5a42c.png)
![R_1=5.21\times 10^{-4}\times [A]^x](/tpl/images/0384/0720/5f894.png) ...[1]
...[1]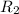
![R_2=K_2\times [A']^x](/tpl/images/0384/0720/6a78b.png)
![R_2=7.42\times 10^{-3}\times [A']^x](/tpl/images/0384/0720/658af.png) ...[2]
...[2]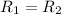 (given)
(given)![5.21\times 10^{-4}\times [A]^x=7.42\times 10^{-3}\times [A']^x](/tpl/images/0384/0720/ebaac.png)
![(\frac{[A]}{[A']})^x=\frac{7.42\times 10^{-3}}{5.21\times 10^{-4}}](/tpl/images/0384/0720/a9fcc.png)