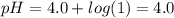Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, kellywelly82
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
Aph 4 buffer solution is prepared by dissolving one mole of a weak acid ha (pka = 4.0) and one mole...
Questions in other subjects:

Mathematics, 11.10.2020 20:01




Mathematics, 11.10.2020 20:01


Chemistry, 11.10.2020 20:01

English, 11.10.2020 20:01

History, 11.10.2020 20:01

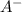 )-
)-![pH=pK_{a}(HA)+log\frac{[A^{-}]}{[HA]}](/tpl/images/0383/9401/34f32.png)
![[A^{-}]](/tpl/images/0383/9401/fe74d.png) and [HA] represents concentration (in molarity) of
and [HA] represents concentration (in molarity) of 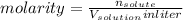
![\frac{[A^{-}]}{[HA]}=\frac{\frac{n_{A^{-}}}{V_{solution}in liter}}{\frac{n_{HA}}{V_{solution}in liter}}=\frac{\frac{1 mol}{10 L}}{\frac{1mol}{10L}}=1](/tpl/images/0383/9401/ac2ca.png)