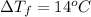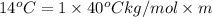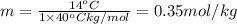Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Calculate the molality of isoborneol in the product if, a) the melting point of pure camphor is 179°...
Questions in other subjects:





Mathematics, 23.06.2019 10:00



Computers and Technology, 23.06.2019 10:00

Mathematics, 23.06.2019 10:00

English, 23.06.2019 10:00

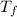 = 165°C
= 165°C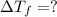
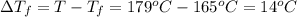
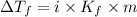
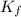 = The freezing point depression constant
= The freezing point depression constant