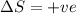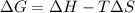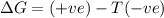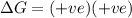Chemistry, 20.11.2019 23:31 annjetero2oy23ay
Consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction is and the enthalpy change is so at a very high temperature, this reaction is probably consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction is and the enthalpy change is so at a very high temperature, this reaction is probably unfavorable; unfavorable; nonspontaneous unfavorable; favorable; spontaneous favorable; unfavorable; spontaneous favorable; unfavorable; nonspontaneous unfavorable; unfavorable; spontaneous

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1


You know the right answer?
Consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction i...
Questions in other subjects:


Mathematics, 15.10.2020 14:01

Mathematics, 15.10.2020 14:01



Mathematics, 15.10.2020 14:01

Physics, 15.10.2020 14:01



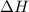 for Endothermic reaction is positive and
for Endothermic reaction is positive and 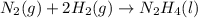
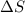 is negative as the randomness decreases when gases convert into liquid.
is negative as the randomness decreases when gases convert into liquid. 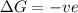 and favourable conditions are
and favourable conditions are 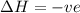 and
and