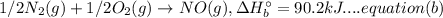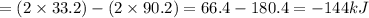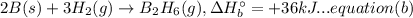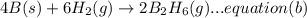Chemistry, 20.11.2019 23:31 headshotplayzcod
A. calculate the enthalpy of the reaction 2no(g)+o2(g)→2no2(g) given the following reactions and enthalpies of formation: 12n2(g)+o2(g)→no2(g), δh∘a=33.2 kj 12n2(g)+12o2(g)→no(g), δh∘b=90.2 kj express your answer with the appropriate units. b. calculate the enthalpy of the reaction4b(s)+3o2(g)→2b2o3(s)given the following pertinent information: b2o3(s)+3h2o(g)→3o2(g)+b2h6(g), δh∘a=+2035 kj2b(s)+3h2(g)→b2h6(g), δh∘b=+36 kjh2(g)+12o2(g)→h2o(l), δh∘c=−285 kjh2o(l)→h2o(g), δh∘d=+44 kjexpress your answer with the appropriate units.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
A. calculate the enthalpy of the reaction 2no(g)+o2(g)→2no2(g) given the following reactions and ent...
Questions in other subjects:


History, 06.07.2019 04:30


Biology, 06.07.2019 04:30

History, 06.07.2019 04:30

Business, 06.07.2019 04:30

Business, 06.07.2019 04:30



Mathematics, 06.07.2019 04:30