
Chemistry, 20.11.2019 21:31 smithsa10630
A40.0-ml solution contains 0.019 m barium chloride (bacl2). what is the minimum concentration of sodium sulfate (na2so4) required in the solution to produce a barium sulfate (baso4) precipitate? the solubility product for barium sulfate is ksp = 1.1 ✕ 10−10.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, ian2006huang
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
A40.0-ml solution contains 0.019 m barium chloride (bacl2). what is the minimum concentration of sod...
Questions in other subjects:


Mathematics, 04.05.2021 07:10


Mathematics, 04.05.2021 07:10

Biology, 04.05.2021 07:10


Mathematics, 04.05.2021 07:10

Mathematics, 04.05.2021 07:10

Mathematics, 04.05.2021 07:10

History, 04.05.2021 07:10

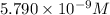 .
.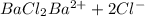
![[BaCl_2]=0.019 M](/tpl/images/0383/3321/1b1d1.png)
![[Ba^{2+}]](/tpl/images/0383/3321/77893.png)
![[Ba^{2+}]=[BaCl_2]=0.019 M](/tpl/images/0383/3321/edb0a.png)

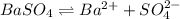
![K_{sp}=[Ba^{2+}]\times S](/tpl/images/0383/3321/9350a.png)
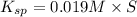
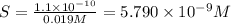
![[SO_4^{2-}]=5.790\times 10^{-9} M](/tpl/images/0383/3321/69505.png)

 of sodium sulfate
of sodium sulfate

