
Chemistry, 20.11.2019 21:31 adriannacomrosenbark
Suppose of lead(ii) nitrate is dissolved in of a aqueous solution of ammonium sulfate. calculate the final molarity of lead(ii) cation in the solution. you can assume the volume of the solution doesn't change when the lead(ii) nitrate is dissolved in it. be sure your answer has the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2

Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 04:00, josephicarusmarrujo
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
You know the right answer?
Suppose of lead(ii) nitrate is dissolved in of a aqueous solution of ammonium sulfate. calculate the...
Questions in other subjects:





History, 10.10.2020 18:01


Mathematics, 10.10.2020 18:01



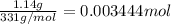
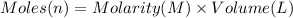
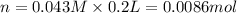
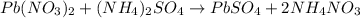
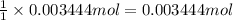 of ammonium sulfate
of ammonium sulfate![[Pb^{2+}]](/tpl/images/0383/3701/0acfd.png)
![[Pb^{2+}]=\frac{0.00 mol}{0.200 L}=0 M](/tpl/images/0383/3701/f1c13.png)


