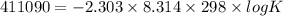Chemistry, 20.11.2019 20:31 pennyluvsu13
Use the tabulated half-cell potentials to calculate the equilibrium constant (k) for the following balanced redox reaction at 25°c. 2 al(s) + 3 mg2+(aq) → 2 al3+(aq) + 3 mg(s) a) 1.1 × 1072 b) 8.9 × 10-73 c) 1.1 × 10-72 d) 1.0 × 1024 e) 4.6 × 1031

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1


Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
Use the tabulated half-cell potentials to calculate the equilibrium constant (k) for the following b...
Questions in other subjects:


Geography, 18.09.2019 12:20


History, 18.09.2019 12:20

Mathematics, 18.09.2019 12:20



Mathematics, 18.09.2019 12:20




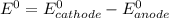
 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Mg^{2+}/Mg]}= -2.37V](/tpl/images/0383/2083/24fc1.png)
![E^0_{[Al^{3+}/Al]}=-1.66V](/tpl/images/0383/2083/0867c.png)
![E^0=E^0_{[Mg^{2+}/Mg]}- E^0_{[Al^{3+}/Al]}](/tpl/images/0383/2083/0b323.png)


 = gibbs free energy
= gibbs free energy