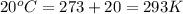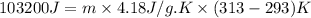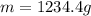Chemistry, 20.11.2019 20:31 bougiehairstudios
For many years drinking water has been cooled in hot climates by evaporating it from the surface of canvas bags or porous clay pots. how many grams of water can be cooled from 40 ∘c to 20 ∘c by the evaporation of 43 g of water? (the heat of vaporization of water in this temperature range is 2.4 kj/g. the specific heat of water is 4.18 j/g⋅k.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, suzymott1562
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3
You know the right answer?
For many years drinking water has been cooled in hot climates by evaporating it from the surface of...
Questions in other subjects:




World Languages, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

History, 18.03.2021 02:40

History, 18.03.2021 02:40


Arts, 18.03.2021 02:40

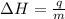
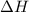 = enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g
= enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g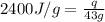
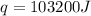
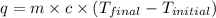
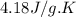
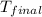 = final temperature =
= final temperature = 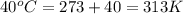
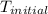 = initial temperature =
= initial temperature =