
Chemistry, 20.11.2019 20:31 11needhelp11
The reaction between so2 and o2 to give so3 was studied to determine the rate law for the reaction. the following results were obtained the rate law is a) k[so2][o2] b) k[so2]2[o2] c) k[so2]2 d) k[so2][ o2]−2 e) k[so2][ o2]−1

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1
You know the right answer?
The reaction between so2 and o2 to give so3 was studied to determine the rate law for the reaction....
Questions in other subjects:





Computers and Technology, 11.07.2019 15:00



History, 11.07.2019 15:00


![\text{Rate}=k[SO_2]^2[O_2]](/tpl/images/0383/2303/0a7d8.png)
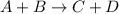
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0383/2303/10aeb.png)
![[A]](/tpl/images/0383/2303/6aa06.png) and
and ![[B]](/tpl/images/0383/2303/db909.png) = concentration of A and B reactant
= concentration of A and B reactant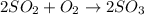
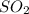 and
and  are the reactants.
are the reactants.![\text{Rate}=k[SO_2]^2[O_2]^1](/tpl/images/0383/2303/d6dd8.png)


